Abstract
INTRODUCTION: Idiopathic neutropenia (IN) of childhood is a benign, self-limiting disorder usually occurring in the first 3 years of life that differs from primary autoimmune neutropenia (AIN) because anti neutrophil antibodies are not detected on repeated indirect testing over time(1). Chronic idiopathic neutropenia (CIN) is a well characterized disorder of elderly with a peculiar immunological pattern(2). Pediatric AIN patients, with atypical features represented by longer disease duration (Long Lasting) or diagnosed later (Late Onset), were recently shown by our group to display a different immune-hematological profile vs typical primary AIN(3). Atypical pediatric IN subjects, i.e. diagnosed later or with longer disease duration, though occasionally reported, were never systematically investigated. In the present study we analyzed a cohort of young patients with atypical IN and AIN, diagnosed after the age of 3 years or with longer disease duration, aiming to identify an immunological signature that might predict their different outcome from classical AIN and IN of infancy.
PURPOSE OF THE STUDY: to analyze a cohort of patients affected with AIN and IN rising > 3 years of age and with duration >12 months or rising <3 years of age but persisting over 36 months.
MATERIALS AND METHODS: Clinical, immunological and genetic data (NGS panel of 160 immunodeficiency/disimmunity genes) of eligible patients were collected from the database of the Italian Neutropenia Registry.
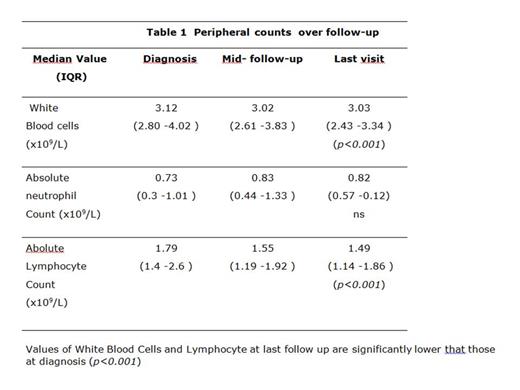
RESULTS: From 2005 to 2020, data from 46 patients (24F, 24 autoimmune and 22 Idiopathic Neutropenia) were retrieved. Median age at onset was 11.2 years (IQR13.2-16.7), median follow-up was 4.3 years (IQR 3.2-6.8). Neither autoimmunity nor additional cytopenias to neutropenia were present at onset. Cumulative incidence at 5 years of autoimmune manifestations (thyroiditis, arthritis, vitiligo, recurrent skin rash) was 11.8% (CI 95% 4.3-30). Throughout the follow up, infections occurred in 32/46 (70%) subjects and in only 16% were severe (meningitis, recurrent pneumonia, sepsis and pericarditis). Infections sites were upper respiratory (in 84% of subjects mouth and gums (47%), and skin (40%) ear (25%), lung (19%) and urinary tract (12%). Fever of unknown origin was detected in 40% of patients. Recurrent infections (more than 3/y) involved mouth (85%) and ear (88%). White Blood Cell, Absolute Neutrophil and Lymphocyte counts retrieved at the beginning of the study, in the middle and at the end of follow up are shown in Table 1. Leucocytes and Lymphocytes at the end of follow up were significantly lower than values seen at diagnosis (p < 0.001) whereas neutrophil count remained stable (p=ns). One third of the cohort had lower values than normal of CD19+ and CD3-CD56+CD16+ cells. B switched memory CD27+/IgD-/IgM- (in 94%) were lower, while marginal zone B lymphocytes CD27+/IgD+/IgM+ (62%) and Tγδ cells (70%) were increased than the normal population according to a pattern comparable to that described in chronic idiopathic neutropenia (CIN). Immunoglobulin serum levels (IgA, IgG and IgM) were below the normal value in 7.5% of the population, but specularly a small subset (7.5%) of IN showed an increase of IgM was seen, similar to what already descibed in adult CIN. The genetic study carried out in 32 patients showed in 5 (16%) pathogenic variants: of immunodeficit/dysregulation (2 TACI, 1 TINF2, 1 CARD11) and in 9 cases (28%) VUS in genes of the same groups (1CARD11, 4CASP10,1DDX41/SOCS1, 1TSR2/DCLRE1c, 1PIK3D, 1TERT/TACI).
CONCLUSIONS: Atypical IN and AIN (of longer duration or occurring in advanced pediatric age) are different from those appearing in early infancy. They seem to display dysimmune/autoimmune features that is some case is proven to rely on a genetic dysimmune background. They might represent an anticipation of more complex autoimmune disorders which may fully manifest later in life. Interstingly these patients seem to share some features with CIN of adults. Definitive conclusions will be drawn by applying more comprehensive genetic nalysis (WES and WGS) on larger group of subjects.
References:
1. Farruggia et al, Am J Hematol. 2019, 94:216-222
2. Mavroudi I et al, Clin Immunol. 2017 Oct;183:75-84
3. Fioredda F et al, Blood Adv 2020 Nov 24;4:5644-5649
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal